X

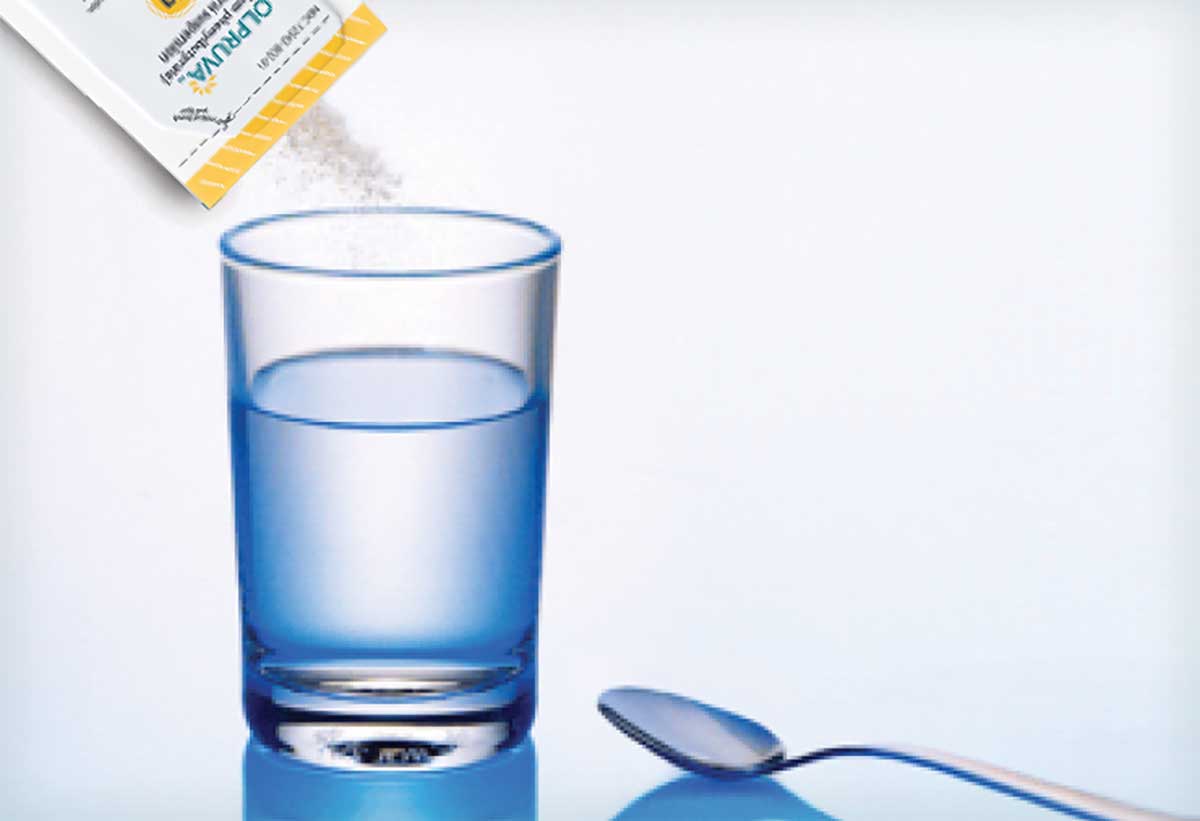
The active ingredient of OLPRUVA is covered by a seal coating and an outer polymer coating.

I refuse to let my urea cycle disorder control my life. It is simply part of my existence.
The US Food and Drug Administration (FDA) approved OLPRUVA: